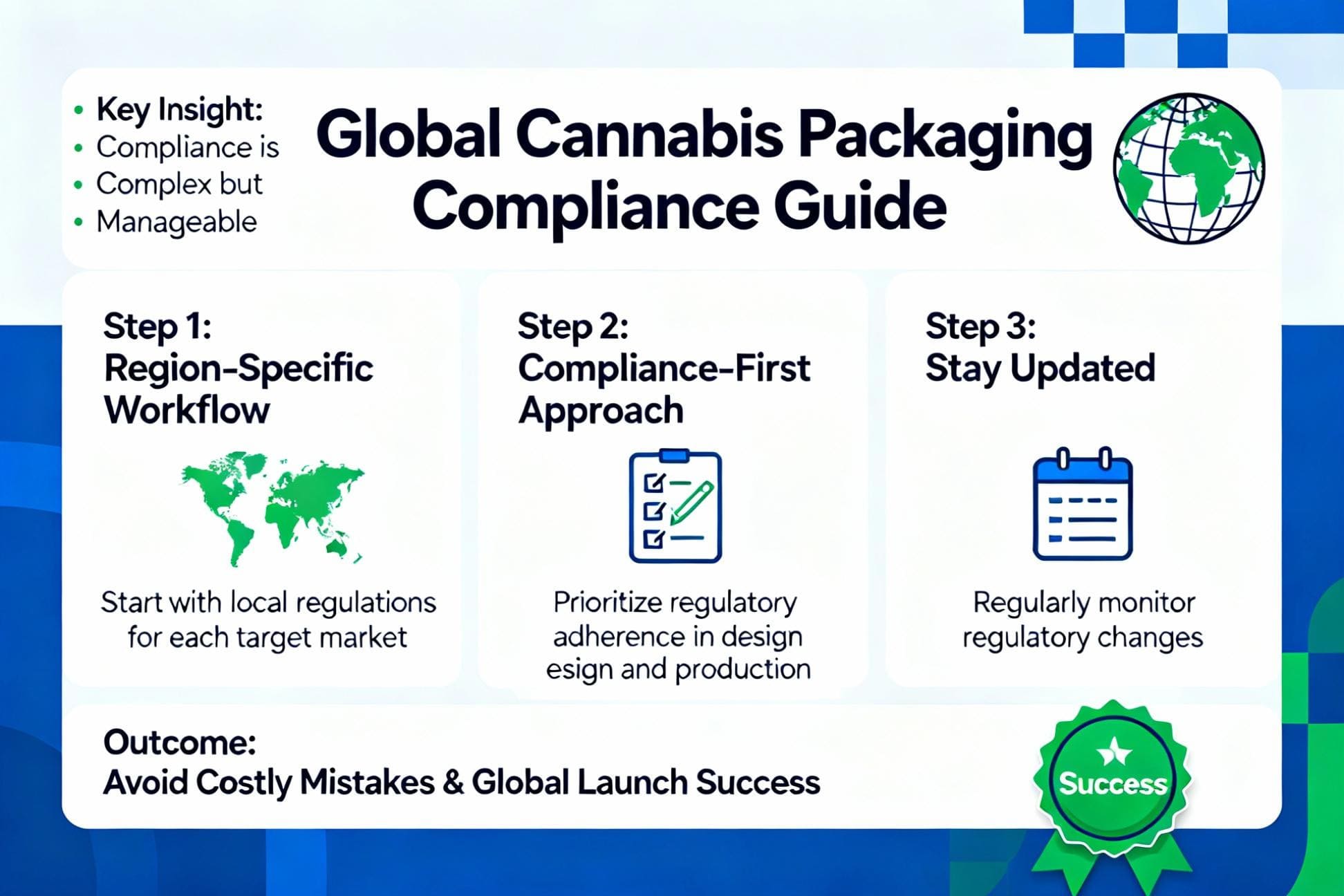
How Can Cannabis Brands Comply With Global Packaging Regulations?
You're launching a cannabis product internationally, but every country has different packaging rules. One small mistake in Canada could mean your entire shipment is rejected in Germany, costing you a fortune.
To comply with global cannabis packaging regulations1, brands must adopt a region-specific strategy. This involves understanding the core requirements for child resistance2, labeling3, and warnings4 for each target market before starting the design process.
I remember a client who was incredibly excited about their new line of vape pens. They had designed this beautiful, minimalist packaging that looked amazing. They spent a fortune on production. Then they tried to ship it to Canada. It was a disaster. The packaging was missing the specific French-language warnings, the THC symbol5 was the wrong size, and the child-resistant mechanism wasn't on Canada's approved list. We had to repackage the entire order, which delayed their launch by three months and cost them tens of thousands of dollars. That experience taught me a hard lesson: with cannabis, compliance isn't a detail you add at the end; it has to be the foundation of your entire packaging design.
What Are the Key Differences in International Cannabis Packaging Rules?
You want to sell your product in multiple countries, but the rules are a confusing mess. North America's requirements seem completely different from Europe's, making a single packaging solution feel impossible.
The main differences are in child-resistance standards, warning symbol specifics, and plain packaging laws6. For example, Canada mandates specific THC symbols and plain packaging, while many US states have their own unique requirements.
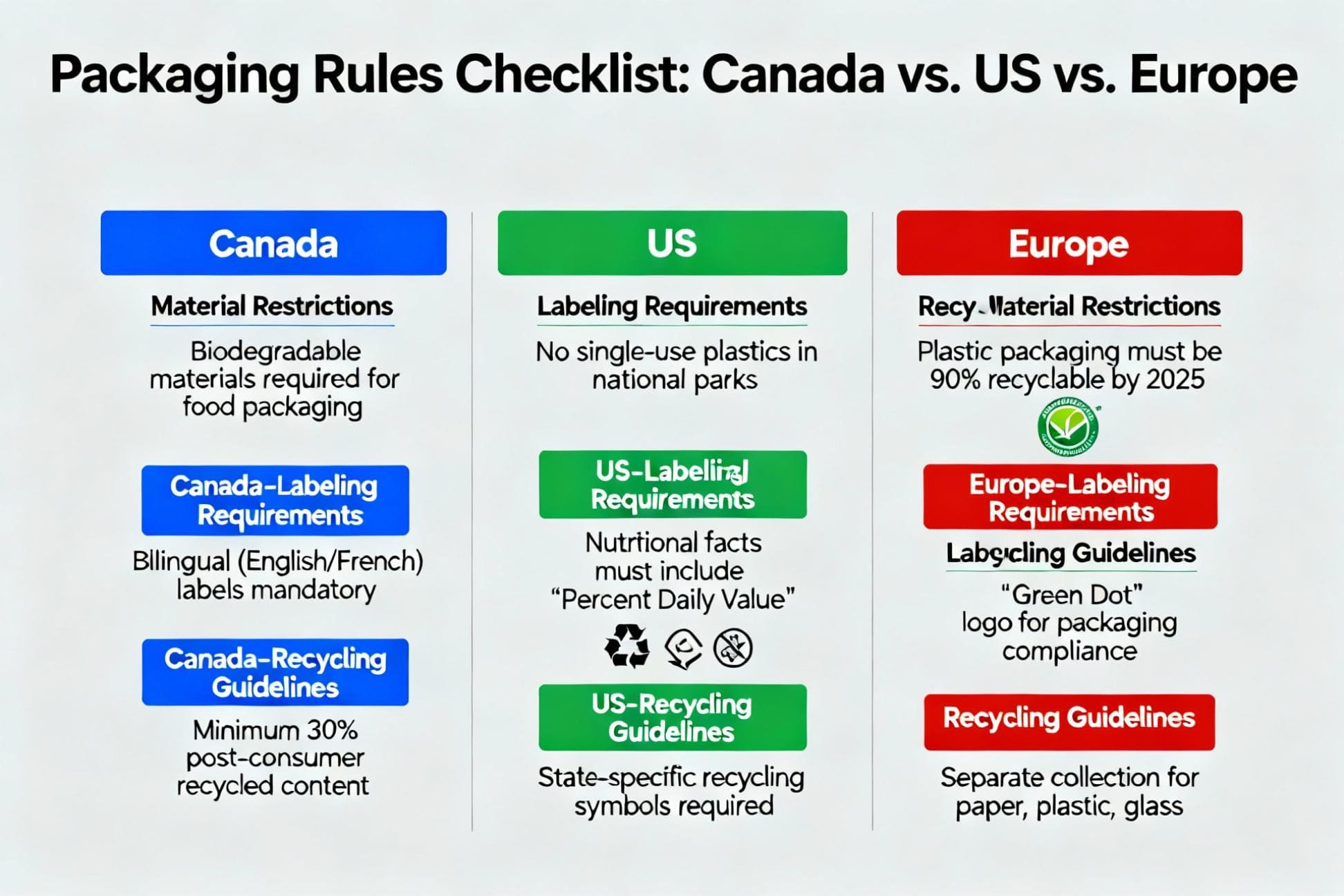
Navigating these differences requires a detailed, region-by-region approach. You simply cannot design one-size-fits-all packaging. A designer like Jacky needs to treat each market as a unique project with its own set of rules. For a quick global cannabis packaging regulations summary, North America is heavily focused on state/province-level rules, with strict child-resistance (CR) and detailed THC labeling. Europe is more fragmented, with Germany focusing on medical-grade standards and others still developing rules. Australia has some of the strictest regulations in the world, leaning heavily on plain packaging inspired by their tobacco laws. The key is to start every project with a compliance checklist7 for your target market and get legal sign-off before you even think about colors or logos. This proactive approach saves incredible amounts of time and money.
Global Compliance Checklist: Key Regional Differences
| Feature | North America (e.g., Canada, California) | Europe (e.g., Germany, Netherlands) | Australia (Medical Market) |
|---|---|---|---|
| Child-Resistance | Mandatory & highly specific (e.g., CFR Title 16, Part 1700). | Generally required, often follows pharmaceutical standards. | Mandatory and very strict, following TGA guidelines. |
| Warning Symbols | Mandatory & standardized. Canada has a red THC stop sign. CA has a "!". | Varies by country. Often text warnings are more common than graphic symbols. | Text-based warnings are required. No promotional imagery. |
| Labeling | Extremely detailed: THC/CBD content, batch number, health warnings8, etc. | Focus on medical information: dosage, patient details, and ingredients. | Strict medical labeling: patient name, doctor, pharmacy, dosage info. |
| Plain Packaging | Common in Canada (drab colors, restricted fonts). Varies in the US. | Not widely adopted yet, branding is generally allowed. | Mandatory. Packaging must be plain, with no logos or branding. |
What Common Compliance Mistakes Should You Avoid?
You’ve submitted your final packaging design for review, feeling confident. Then it gets rejected for a tiny detail you overlooked, like the font size on a warning label being half a millimeter too small.
The most common mistakes are incorrect warning symbols, wrong font sizes for health warnings, missing potency information9 (THC/CBD content), and using uncertified child-resistant mechanisms10. These errors often lead to costly recalls11.

Details are everything in regulated packaging. I’ve seen brands fail compliance because the space between two lines of warning text was incorrect or because they used the wrong pantone color for a mandated symbol. It sounds trivial, but regulators are not flexible. One of the most frequent and costly errors is improper THC labeling. Some regions require potency per serving AND per package. Others require the universal THC symbol to be a specific size relative to the rest of the label. Another major pitfall is the child-resistant (CR) certification. A mechanism might be CR-certified in the United States, but that doesn't mean it's automatically accepted in Canada or Germany. You must verify that your chosen CR solution is certified for the specific region you're selling in. Always double-check every single detail against the official government regulations before going to print.
Top 3 Compliance Pitfalls
- Incorrect Warning Labels: This includes using the wrong symbol for the region, having the wrong dimensions, or placing it incorrectly on the package. Every detail matters.
- Illegible Text: Health warnings and ingredient lists often have minimum font size requirements12. If your text is too small, your product will be non-compliant.
- Inaccurate Potency Declaration: The THC and CBD content must be clearly stated, often in both milligrams and percentage. Any error here is a major red flag for regulators.
How Can You Stay Updated With Rapidly Changing Laws?
You finally master the regulations for your target market. Then, six months later, the government updates the rules, and your packaging is suddenly obsolete and non-compliant. It feels like you're always one step behind.
Regularly monitor the official websites of government regulatory bodies13 in your target markets. Subscribing to industry newsletters and consulting with a compliance expert14 are also essential strategies for staying current.

The cannabis industry is new, and its laws are constantly evolving. You cannot rely on old information. The only way to stay safe is to go directly to the source. Your design team should have bookmarks for the primary regulatory agencies in every country you operate in. For example, if you sell in Canada, your team needs to check Health Canada’s cannabis regulations page frequently. For the US, you need to monitor the individual state cannabis control boards, as rules vary wildly from state to state. This isn’t a one-time task; it should be part of a monthly or quarterly review process. Assign someone on your team to be the "compliance champion" whose job it is to track these changes and update your internal design guides. This proactive monitoring prevents you from being caught off guard by a sudden rule change.
Key Regulatory Websites to Monitor
- Canada: Health Canada - Cannabis section
- United States: Food and Drug Administration (FDA) for general guidance, plus individual State Cannabis Control Board websites (e.g., California's Department of Cannabis Control).
- Australia: Therapeutic Goods Administration (TGA).
- Germany: Federal Institute for Drugs and Medical Devices (BfArM).
How Can You Build a Compliance-First Packaging Workflow?
Your design team creates amazing concepts, but they are often rejected by the legal team late in the process. This causes frustrating delays, wasted effort, and friction between departments.
Integrate a compliance check at the very beginning of your workflow, before significant design work begins. The legal or compliance expert should provide a clear design brief that outlines all regulatory constraints from day one.
<sup id=](https://whatapackaging.com/wp-content/uploads/2025/10/28-5.jpg) 15 happens at the start, not the end" title="A Compliance-First Packaging Design Workflow" />
15 happens at the start, not the end" title="A Compliance-First Packaging Design Workflow" />
The traditional workflow where design works in a silo and then hands off to legal for a final "yes" or "no" is broken. It's inefficient and expensive. A better approach is to make compliance a creative partner. Before your designer even opens their software, they should receive a "compliance guardrail" document. This document, prepared by a legal expert, clearly defines the non-negotiables: required text, symbol specifications, mandatory empty space, color restrictions, and approved CR mechanisms. This way, the designer’s creativity is channeled within the boundaries of what is legal. Your manufacturing partner16 also plays a crucial role. We always work with our clients to confirm that the materials and structures they choose are compatible with the required CR features and printing needs for compliance. This collaborative, upfront approach turns compliance from a barrier into just another part of the design problem to be solved.
Steps for a Compliance-First Workflow
- Legal Brief: Legal/compliance provides a detailed brief with all rules before design starts.
- Concept Check: Designer creates initial concepts within these rules. A quick legal check happens here.
- Detailed Design: The chosen concept is fully designed with all regulatory text and symbols.
- Final Legal Sign-Off: Legal reviews the final, print-ready artwork for any errors.
- Factory Verification: The factory confirms they can produce the CR feature and print all details accurately.
Conclusion
Global cannabis packaging compliance is complex but manageable. By starting with a region-specific, compliance-first workflow and staying updated on regulations, your brand can avoid costly mistakes and launch successfully worldwide.
Understanding global cannabis packaging regulations is crucial for compliance and successful international product launches. ↩
Child resistance is a key factor in cannabis packaging; learn the specific requirements to ensure safety and compliance. ↩
Proper labeling is essential for compliance; explore the specific requirements for different regions. ↩
Warning labels are critical for consumer safety; find out what is required in your target market. ↩
The THC symbol is a regulatory requirement; understanding its specifications can prevent costly mistakes. ↩
Plain packaging laws vary by region; knowing these can help you design compliant packaging. ↩
A compliance checklist is vital for ensuring all regulations are met; learn what to include. ↩
Health warnings are essential for consumer safety; find out the specific requirements for your market. ↩
Accurate potency information is crucial for compliance; explore the requirements for your products. ↩
Using certified mechanisms is essential for compliance; explore the options available. ↩
Understanding recall causes can help you avoid them; learn the common pitfalls in cannabis packaging. ↩
Font size requirements are critical for compliance; learn the specifics to avoid rejections. ↩
Knowing the regulatory bodies helps you stay updated on compliance requirements and changes. ↩
A compliance expert can provide invaluable guidance to navigate complex regulations effectively. ↩
Legal review is essential to ensure compliance; understand its role in the design process. ↩
A good manufacturing partner can ensure compliance; find out how they can support your packaging needs. ↩